A team of scientists at the Pacific Northwest National Laboratory of the Department of Energy are working together with researchers from the Wuhan University in China to manufacture electrodes using nanomaterials that can function well with sodium.
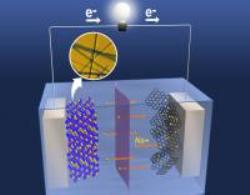
The electrodes in lithium rechargeable batteries consist of manganese oxide. When batteries are charged or in use, the atoms present in this metal oxide form numerous tunnels and holes and enable the free movement of lithium ions. The free motion of lithium ions allows the battery to either retain power or release it. Replacing the lithium ions with sodium ions is challenging. Sodium ions are 70% larger than lithium ions and do not accommodate well in the crevices.
Researchers tried to make larger holes in manganese oxide with the use of nanomaterials. These materials are about a million times smaller than a dime.
The team combined different types of atomic building blocks of manganese oxide of which block had atoms that arranged themselves in pyramids and the other block atoms that formed an octahedron and predicted that the resultant material would have huge S-shaped tunnels and little five-sided tunnels for ions to pass. Following the mixing, the team subjected the materials to temperatures from 450°C to 900°C. Next, they observed the materials and evaluated the most effective type of treatment.
With the help of a scanning electron microscope, the team found that the quality of material differed at different temperatures. When manganese oxide was treated at 750°C, it created the most effective crystals. When heated to 600°C, the nanowires featured pockmarks that could obstruct the sodium ions, but the 750°C-treated wires appeared even and crystalline.
The electrode was dipped in electrolyte comprising sodium ions enabling the electrodes to generate a current. They charged and discharged the new battery cells continually. The peak capacity was recorded as 128 mA/g of electrode in the coursework of discharge of the new battery cell.
Finally, the team charged the experimental battery cell at various speeds to choose the time it takes to take up electricity. The faster the battery got charged, the lesser electricity it could retain. Thus, it was established that the rate at which sodium ions diffused in to the manganese oxide restricted the capacity of the battery cell.
No comments:
Post a Comment