the wunderkind nanotechnology in pharmaceutics: Creating multifunctional nanocarriers.
The last few years saw tremendous progress in the use of nanoparticles to enhance the in vivo efficiency of many drugs. Currently used pharmaceutical nanocarriers, such as liposomes, micelles, nanoemulsions, polymeric nanoparticles and many others demonstrate a broad variety of useful properties, such as for instance increased longevity in the blood, specific targeting to certain disease sites, or enhanced intracellular penetration. Some of these pharmaceutical carriers have already made their way into clinics, while others are still under preclinical development. In the next phase of developing nanocarriers, researchers are intrigued by the possibility to synthesize pharmaceutical nanocarriers that possess not only one but several properties. Such particles can significantly enhance the efficacy of many therapeutic and diagnostic protocols. A brandnew review paper considers current status and possible future directions in the emerging area of multifunctional nanocarriers with primary attention on the combination of such properties as longevity, targetability, intracellular penetration and contrast loading.
Vladimir P. Torchilin, Distinguished Professor of Pharmaceutical Sciences and Director of the Center for Pharmaceutical Biotechnology and Nanomedicine at Northeastern University, described to Nanowerk how such nanocarriers would work: "One may want to have a drug-loaded nanocarrier demonstrating the following set of properties: (a) prolonged circulation in the blood; (b) ability to accumulate – specifically or non-specifically – in the required pathological zone, (c) responsiveness to local stimuli, such as pH and/or temperature changes, resulting, for example, in accelerated drug release, (d) allow for an effective intracellular drug delivery and further to individual cell organelles, and (e) bear a contrast/reporter moiety allowing for the real-time observation of its accumulation inside the target. Some other, more exotic properties can be added to the list, such as magnetic sensitivity."
In order to prepare such a smart multifunctional pharmaceutical nanocarrier, chemical moieties providing certain required individual properties have to be simultaneously assembled on the surface of the same nanoparticle. Moreover, these individual moieties have to function in a certain coordinated way to provide a desired combination of useful properties.
Torchilin cautions that systems like these still represent quite a challenge to researchers.
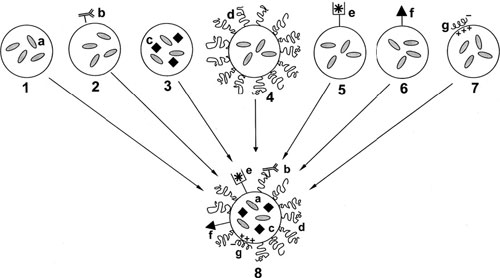
The schematic structure of the assembly of the multifunctional pharmaceutical nanocarrier. 1 – Traditional “plain” nanocarrier (a – drug loaded into the carrier); 2 – targeted nanocarrier or immunocarrier (b – specific targeting ligand, usually a monoclonal antibody, attached to the carrier surface); 3 – magnetic nanocarrier (c – magnetic particles loaded into the carrier together with the drug and allowing for the carrier sensitivity towards the external magnetic field and its use as a contrast agent for magnetic resonance imaging); 4 – long-circulating nanocarrier (d – surface-attached protecting polymer (usually PEG) allowing for prolonged circulation of the nanocarrier in the blood); 5 – contrast nanocarrier for imaging purposes (e – heavy metal atom – 111In, 99mTc, Gd, Mn – loaded onto the nanocarrier via the carrier-incorporated chelating moiety for gamma- or MR imaging application); 6 – cell-penetrating nanocarrier (f – cell-penetrating peptide, CPP, attached to the carrier surface and allowing for the carrier enhanced uptake by the cells); 7 – DNA-carrying nanocarrier such as lipoplex or polyplex (g – DNA complexed by the carrier via the carrier surface positive charge); 8 – hypothetical multifunctional pharmaceutical nanocarrier combining the properties of the carriers # 1–7. (Reprinted with permission from Elsevier)
Multifunctional nanocarriers need to possess a number of basic properties to make them effective and efficient:
Longevity in the blood
Nanoparticles are normally attacked as foreign substance by the body's defense system and removed from circulation long prior to completion of their function. Thus, the basic property of any multifunctional nanocarrier is its longevity, and long-circulating pharmaceuticals and pharmaceutical carriers represent currently an important and still growing area of biomedical research.
Chemical modification of pharmaceutical nanocarriers with certain synthetic polymers, such as polyethylene glycol (PEG), is the most frequent way to impart the in vivo longevity to drug carriers. The term “steric stabilization” has been introduced to describe the phenomenon of polymer-mediated protection. On the biological level, coating nanoparticles with PEG sterically hinders interactions of blood components with their surface and reduces the binding of plasma proteins with PEGylated nanoparticles. This prevents drug carrier interaction with opsonins and slows down their fast capture by the reticuloendothelial system (RES).
Several other polymers have also been suggested as alternative steric protectors for nano drug carriers and there is a lot of ongoing research in this area. These polymers are expected to be biocompatible, soluble, hydrophilic, and with a highly flexible main chain.
In summary, the most significant biological consequence of nanocarrier modification with protecting polymers is the sharp increase in its circulation time and decrease in their RES accumulation.
Targetability
To increase the functionality of pharmaceutical nanocarriers involves adding the property of the specific target recognition to the carrier's ability to circulate long, i.e. simultaneously attach both the protecting polymer and the targeting moiety on the surface of the nanocarrier. Targeting of drug carriers with the aid of ligands specific to cell surface-characteristic structures allows for the selective drug delivery to those cells. To obtain “simple” targeted nanocarriers, a variety of methods have been developed to attach corresponding vectors (antibodies, peptides, sugar moieties, folate, and other ligands) to the carrier surface.
Stimuli sensitivity
An additional function that researchers are keen to add to long-circulating PEGylated pharmaceutical carriers will allow for the detachment of protecting PEG chains under the action of certain local stimuli characteristic of pathological areas, such as decreased pH value or increased temperature usually noted for inflamed areas.
The problem here is that the stability of PEGylated nanocarriers may not always be favorable for drug delivery. For instance, if drug-containing nanocarriers accumulate inside a tumor, they may be unable to easily release the drug to kill the tumor cells. In order to solve these problems, for example, in the case of long-circulating liposomes, the chemistry was developed to detach PEG from the lipid anchor in the desired conditions.
As a result, polymeric components with pH-sensitive (pH-cleavable) bonds are used to produce stimuli-responsive drug delivery systems that are stable in the circulation or in normal tissues, however, acquire the ability to degrade and release the entrapped drugs in body areas or cell compartments with lowered pH, such as tumors, infarcts, or inflammation zones.
Intracellular delivery
Many biologically active compounds, including macromolecular drugs, need to be delivered intracellularly, for instance for gene therapy, to exert their therapeutic action inside the cell onto nucleus or other specific organelles, such as mitochondria. However, the lipophilic nature of the biological membranes restricts the direct intracellular delivery of such compounds.
Current delivery systems, be they viral or non-viral, all have drawbacks, such as for instance non-specificity and cytotoxic reactions, which makes them quite ineffective for clinical use. Researchers therefore have focused on the development of a new method that can deliver genetic constructs directly into the cytoplasm of the target cells. These include: the application of bimetallic nanorods that can simultaneously bind compacted DNA plasmid and targeting ligands in a spatially defined manner; membrane-destabilizing lipid components and anionic polymers; functionalizing drugs with proteins and peptides that demonstrate a unique ability to penetrate into cells (“protein transduction” phenomenon) and therefore may serve as a "transport" through the cell membrane.
Contrast moiety for visualization
To make it possible to use pharmaceutical nanocarriers for diagnostic/imaging purposes as well as to allow for their real-time biodistribution and target accumulation, contrast reporter moieties can be added to multifunctional nanocarriers to enable imaging modalities such as magnetic resonance, computer tomography or ultra-sonography.
Nanocarriers are able to carry multiple contrast moieties for an efficient delivery of contrast agents to areas of interest and enhancing a signal from these areas. Among nanocarriers for contrast agents, liposomes and micelles draw a special attention because of their easily controlled properties and good pharmacological characteristics. For instance, liposomes may incorporate contrast agents in both internal aqueous compartment and membrane.
Unlimited opportunities?
"As clearly follows from these examples, preparing multifunctional nanocarriers with controlled properties require the conjugation of proteins, peptides, polymers, cell-penetrating moieties, reporter groups and other functional ligands to the carrier surface; although, in certain cases, functional components may be loaded inside the nanocarrier or distributed within the nanocarrier structure" Torchilin explains. "This attachment can proceed non-covalently, via the hydrophobic adsorption of certain intrinsic or specially inserted hydrophobic groups in the ligands to be attached onto or into the surface of the nanocarrier. More frequently, the attachment is performed chemically, via the interaction of reactive groups generated on the carrier surface and certain groups in the molecule to be attached."
"Looking at all these developments, it becomes clear that multifunctional pharmaceutical nanocarriers could provide almost unlimited opportunities in producing highly efficient and specialized systems for drugs, genes, and diagnostic agents" Torchilin concludes. "Such multifunctional delivery systems with their individual functions acting in coordinated way should allow for delivery of pharmaceutical agents with required temporal and spatial deposition and release pattern. Although the approach is just emerging, it shows a promising future."
Vladimir P. Torchilin, Distinguished Professor of Pharmaceutical Sciences and Director of the Center for Pharmaceutical Biotechnology and Nanomedicine at Northeastern University, described to Nanowerk how such nanocarriers would work: "One may want to have a drug-loaded nanocarrier demonstrating the following set of properties: (a) prolonged circulation in the blood; (b) ability to accumulate – specifically or non-specifically – in the required pathological zone, (c) responsiveness to local stimuli, such as pH and/or temperature changes, resulting, for example, in accelerated drug release, (d) allow for an effective intracellular drug delivery and further to individual cell organelles, and (e) bear a contrast/reporter moiety allowing for the real-time observation of its accumulation inside the target. Some other, more exotic properties can be added to the list, such as magnetic sensitivity."
In order to prepare such a smart multifunctional pharmaceutical nanocarrier, chemical moieties providing certain required individual properties have to be simultaneously assembled on the surface of the same nanoparticle. Moreover, these individual moieties have to function in a certain coordinated way to provide a desired combination of useful properties.
Torchilin cautions that systems like these still represent quite a challenge to researchers.

The schematic structure of the assembly of the multifunctional pharmaceutical nanocarrier. 1 – Traditional “plain” nanocarrier (a – drug loaded into the carrier); 2 – targeted nanocarrier or immunocarrier (b – specific targeting ligand, usually a monoclonal antibody, attached to the carrier surface); 3 – magnetic nanocarrier (c – magnetic particles loaded into the carrier together with the drug and allowing for the carrier sensitivity towards the external magnetic field and its use as a contrast agent for magnetic resonance imaging); 4 – long-circulating nanocarrier (d – surface-attached protecting polymer (usually PEG) allowing for prolonged circulation of the nanocarrier in the blood); 5 – contrast nanocarrier for imaging purposes (e – heavy metal atom – 111In, 99mTc, Gd, Mn – loaded onto the nanocarrier via the carrier-incorporated chelating moiety for gamma- or MR imaging application); 6 – cell-penetrating nanocarrier (f – cell-penetrating peptide, CPP, attached to the carrier surface and allowing for the carrier enhanced uptake by the cells); 7 – DNA-carrying nanocarrier such as lipoplex or polyplex (g – DNA complexed by the carrier via the carrier surface positive charge); 8 – hypothetical multifunctional pharmaceutical nanocarrier combining the properties of the carriers # 1–7. (Reprinted with permission from Elsevier)
Multifunctional nanocarriers need to possess a number of basic properties to make them effective and efficient:
Longevity in the blood
Nanoparticles are normally attacked as foreign substance by the body's defense system and removed from circulation long prior to completion of their function. Thus, the basic property of any multifunctional nanocarrier is its longevity, and long-circulating pharmaceuticals and pharmaceutical carriers represent currently an important and still growing area of biomedical research.
Chemical modification of pharmaceutical nanocarriers with certain synthetic polymers, such as polyethylene glycol (PEG), is the most frequent way to impart the in vivo longevity to drug carriers. The term “steric stabilization” has been introduced to describe the phenomenon of polymer-mediated protection. On the biological level, coating nanoparticles with PEG sterically hinders interactions of blood components with their surface and reduces the binding of plasma proteins with PEGylated nanoparticles. This prevents drug carrier interaction with opsonins and slows down their fast capture by the reticuloendothelial system (RES).
Several other polymers have also been suggested as alternative steric protectors for nano drug carriers and there is a lot of ongoing research in this area. These polymers are expected to be biocompatible, soluble, hydrophilic, and with a highly flexible main chain.
In summary, the most significant biological consequence of nanocarrier modification with protecting polymers is the sharp increase in its circulation time and decrease in their RES accumulation.
Targetability
To increase the functionality of pharmaceutical nanocarriers involves adding the property of the specific target recognition to the carrier's ability to circulate long, i.e. simultaneously attach both the protecting polymer and the targeting moiety on the surface of the nanocarrier. Targeting of drug carriers with the aid of ligands specific to cell surface-characteristic structures allows for the selective drug delivery to those cells. To obtain “simple” targeted nanocarriers, a variety of methods have been developed to attach corresponding vectors (antibodies, peptides, sugar moieties, folate, and other ligands) to the carrier surface.
Stimuli sensitivity
An additional function that researchers are keen to add to long-circulating PEGylated pharmaceutical carriers will allow for the detachment of protecting PEG chains under the action of certain local stimuli characteristic of pathological areas, such as decreased pH value or increased temperature usually noted for inflamed areas.
The problem here is that the stability of PEGylated nanocarriers may not always be favorable for drug delivery. For instance, if drug-containing nanocarriers accumulate inside a tumor, they may be unable to easily release the drug to kill the tumor cells. In order to solve these problems, for example, in the case of long-circulating liposomes, the chemistry was developed to detach PEG from the lipid anchor in the desired conditions.
As a result, polymeric components with pH-sensitive (pH-cleavable) bonds are used to produce stimuli-responsive drug delivery systems that are stable in the circulation or in normal tissues, however, acquire the ability to degrade and release the entrapped drugs in body areas or cell compartments with lowered pH, such as tumors, infarcts, or inflammation zones.
Intracellular delivery
Many biologically active compounds, including macromolecular drugs, need to be delivered intracellularly, for instance for gene therapy, to exert their therapeutic action inside the cell onto nucleus or other specific organelles, such as mitochondria. However, the lipophilic nature of the biological membranes restricts the direct intracellular delivery of such compounds.
Current delivery systems, be they viral or non-viral, all have drawbacks, such as for instance non-specificity and cytotoxic reactions, which makes them quite ineffective for clinical use. Researchers therefore have focused on the development of a new method that can deliver genetic constructs directly into the cytoplasm of the target cells. These include: the application of bimetallic nanorods that can simultaneously bind compacted DNA plasmid and targeting ligands in a spatially defined manner; membrane-destabilizing lipid components and anionic polymers; functionalizing drugs with proteins and peptides that demonstrate a unique ability to penetrate into cells (“protein transduction” phenomenon) and therefore may serve as a "transport" through the cell membrane.
Contrast moiety for visualization
To make it possible to use pharmaceutical nanocarriers for diagnostic/imaging purposes as well as to allow for their real-time biodistribution and target accumulation, contrast reporter moieties can be added to multifunctional nanocarriers to enable imaging modalities such as magnetic resonance, computer tomography or ultra-sonography.
Nanocarriers are able to carry multiple contrast moieties for an efficient delivery of contrast agents to areas of interest and enhancing a signal from these areas. Among nanocarriers for contrast agents, liposomes and micelles draw a special attention because of their easily controlled properties and good pharmacological characteristics. For instance, liposomes may incorporate contrast agents in both internal aqueous compartment and membrane.
Unlimited opportunities?
"As clearly follows from these examples, preparing multifunctional nanocarriers with controlled properties require the conjugation of proteins, peptides, polymers, cell-penetrating moieties, reporter groups and other functional ligands to the carrier surface; although, in certain cases, functional components may be loaded inside the nanocarrier or distributed within the nanocarrier structure" Torchilin explains. "This attachment can proceed non-covalently, via the hydrophobic adsorption of certain intrinsic or specially inserted hydrophobic groups in the ligands to be attached onto or into the surface of the nanocarrier. More frequently, the attachment is performed chemically, via the interaction of reactive groups generated on the carrier surface and certain groups in the molecule to be attached."
"Looking at all these developments, it becomes clear that multifunctional pharmaceutical nanocarriers could provide almost unlimited opportunities in producing highly efficient and specialized systems for drugs, genes, and diagnostic agents" Torchilin concludes. "Such multifunctional delivery systems with their individual functions acting in coordinated way should allow for delivery of pharmaceutical agents with required temporal and spatial deposition and release pattern. Although the approach is just emerging, it shows a promising future."
No comments:
Post a Comment